Genetically Modified Organisms Testing
Genetically Modified Organism (GMO)
GMO’s are created by introducing genetic material from a different organism to give the organism some desirable traits like pesticide resistance, resistance to disease, more yield etc. Resistance against insects is made by introducing into the plant DNA a gene for toxin production from bacillus thuringienesis whilst resistance to virus is by injecting genes from virus that causes the plant disease. Herbicide resistance is achieved through the introduction of a gene from a bacterium conveying resistance to some herbicides. Introduction of such genes from bacteria and viruses have given rise to suspicion of allergenicity, microbial resistance, mutagenicity and general unknown long-term impairment to human health. Foods from genetically modified plants on sale in the US are regulated by the FDA and they must meet the safety standards as traditional foods and they must be tested. In Singapore all research into GMO must comply to reach guidelines for GMO by GMAC, the genetic modification advisory authority. The GMAC controls the release of GMO foods in Singapore. The official regulatory agency for GM foods is the Singapore Food Agency (SFA). According to the International Service for the Acquisition of Agri-biotech Applications (ISAAA), the principal genetically modified (GM) crop in 2012 was GM soybean, followed by GM maize, GM cotton, and GM canola. These are sometimes called first generation GM foods which gives agronomic traits. Second generation GM foods awaiting regulatory approvals gives enhanced nutritional contents like golden rice with pro vitamin A and soya beans with enhanced omega 3 fatty acids. There are no current legislation and labelling requirements for GMO in Singapore but food products like all other foods must meet food labelling requirements to facilitate tracing and recall.
The SFA monitors the presence of GM foods in our market through regular testing in their laboratories. Since the 1st of September 1998 all food products which are containing genetically modified organisms (GMO) must be labelled. As stated by the Council of Ministers of the European Union (EU), regulation for the labelling of Novel Food: Decision No. 1139/98/EC. Furthermore, since April 2004 the EU directive 1829/2003 came into force and determines the labeling threshold value at 0.9 % in EU for food and feed.
The quality of food is becoming increasingly important to many consumers. To ensure the necessary transparency, i.e. the European Council Regulation (EC) (No. 834/2007) on organic production was implemented. This regulation regulates the use and labeling of genetically modified plants in food and feed (European labeling requirements 1829/2003 and 1830/2003). Compliance with this basic organic regulation can be shown by a corresponding seal on the food products. According to national or regional regulations, zero tolerance applies to non-approved GMO events. As such, only qualitative tests are of interest for these products at the moment. The detection limit lies at 0.01 %, depending on the matrix and the application.
Regulation EC 619/2011 exists as a special rule for animal feeds. Provided that certain conditions are met (expired EU approval, approval in the country of origin, availability of tests and reference material, etc.), a technical threshold value of 0.1 % applies for non-approved GMO events. This is particularly significant as not all imports are contamination-free of GMOs and GMO-free maize and soya.
Testing of GMO products
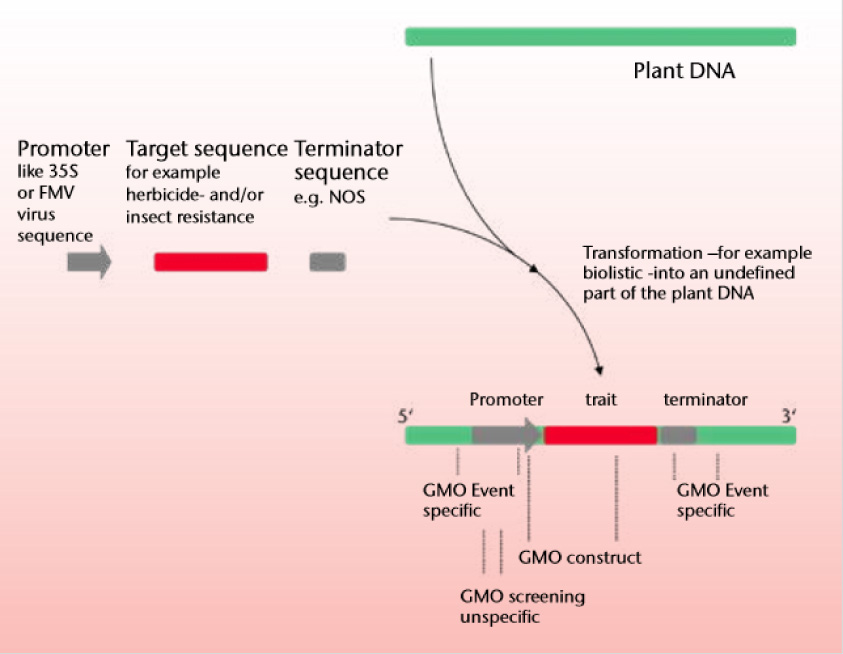
Most testing of GMO products are on crops. For crops there is the question of whether the product lies above the defined threshold of GMO events or mandatory labelling values. In EU, a value of 0.9% applies to the GMO event in the respective plant matrix. Below that coincidental contamination do not need to be declared. Quantification is via the respective genes and then calibrated to convert to % value. Most GMO events contain the promoter/terminator sequences 35S, NOS, FMV, BAR, CTP@:CTP4 EPSPS which are not natural in plants. Identification of these genes are used for absence/presence screening. Some new GMO do not contain these sequences and must be performed with direct identification. R-Biopharm uses a multilevel approach to GMO analysis. First the DNA is extracted using SureFood® DNA PREP Advanced. The sample is next screened for vectors such as 35S, NOS or FMV using SureFood® GMO SCREEN. If it is positive for only 35S then it is checked with GMO screen CaMV to exclude natural virus contamination. The differentiated detection of different vectors helps exclude/include potential GMO events in particular matrixes. The SureFood® GMO SCREEN 4plex 35S/NOS/FMV+IAC (S2126) in parallel or sequentially with BAR/NPTII/PAT/CTP2:CP4 EPSPS (S2127) offers a comprehensive analysis. For food where zero tolerance is needed of unapproved GMO in EU, then a qualitative detection is made using Sure Food GMO ID kit which has a detection level of 0.01% depending on matrix and level of processing. Where GMO is allowed in proportion of 0.9% per matrix, quantification is made using SureFood® GMO QUANT. Corn and soya kits can be used to quantify GMO corn and Soya.
LEARN MORE
Reach Us Today

We provide the best services about science.
About Company
Sitemap
Please contact our friendly sales staff for more information.
Feel free to ask us questions. We would love to assist you !
